
Galvashield® SM-DAS Wins Innovative Product Award
World of Concrete Announces the 2025 Innovative Product Award Winners : The third annual awards …
Welcome to Vector Corrosion Technologies' deep dive into the fascinating and often misunderstood world of corrosion. In this blog, we'll explore why the corrosion of steel in concrete structures is a significant issue, how it occurs, and what can be done to mitigate its effects. Whether you're a civil engineer, a construction professional, or simply curious about the science behind corrosion, this article is for you.
Corrosion is a natural process where metals deteriorate due to reactions with their environment. This is the result of chemical and electrochemical reactions. Concrete corrosion often refers to the deterioration and more specifically the corrosion of the steel reinforcement within the concrete.
Corrosion is an electrochemical reaction, meaning that the reaction includes both electrical and chemical reactions (Figure 1):
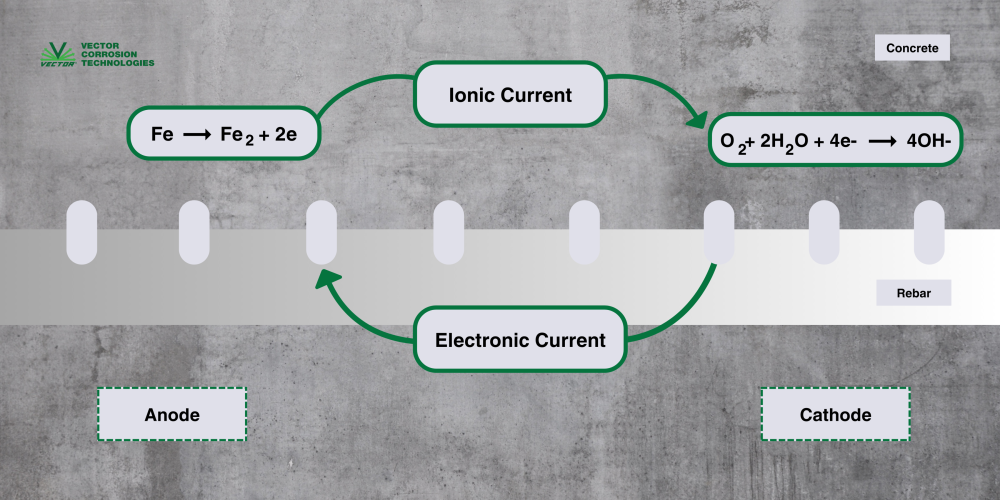
To learn more about concrete durability, watch this YouTube video.
Steel can corrode when solid concrete is contaminated with corrosive compounds or is exposed to reactive liquids and gases. When this occurs, corrosion can initiate.
When steel is embedded in concrete, it develops a passive oxide film due to the high pH of the concrete. The high pH creates an environment that promotes the formation of the passive film, which is basically stable, tightly adhered rust. and acts as a protective layer.
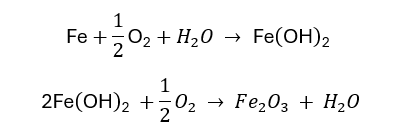
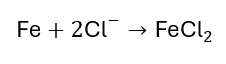
Damage Resulting from Corrosion:
Steel’s natural tendency to corrode and revert to its original, lowest-energy state in the form of iron oxides can result in damage to concrete structures. The expansive byproduct of the corrosion process (formation of rust) causes tensile stress in the concrete cover leading to concrete cracking, delamination, and spalling.
Primary causes of corrosion:
There are other mechanisms that can degrade concrete such as microbes, chemicals, and industrial waste.
Cathodic Protection:
Cathodic protection (CP) is a method of corrosion control that applies a direct current to a metal under protection, forcing it to become a cathode (Figure 2).
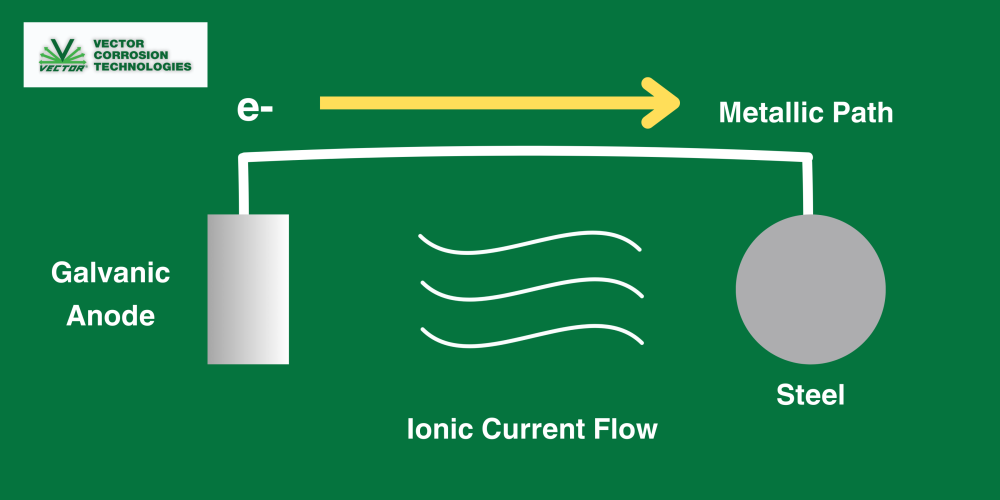
Two Types of Cathodic Protection:
Cathodic Protection (CP) is a method of corrosion control, involving the application of direct current to the protected metal, forcing it to become a cathode.
Explore our range of cathodic protection systems here.
Watch to learn why it’s important to Not Patch it but Repair It.
Corrosion affects concrete structures globally, resulting in annual costs in the billions.
Steel corrosion in concrete is accelerated in severe conditions, particularly those with high salt levels or extreme temperatures.
Steel production accounts for 27% of worldwide manufacturing carbon dioxide emissions and 10.5% of total global carbon dioxide emissions. Replacement of corroded steel accounts for 1.6–3.4% of all CO2 emissions.
Are you weighing your options between a rebuild or a repair? Check out our Environmental Impact Calculator to understand the effects your project has on our environment.
Reinforcing steel corrosion is the primary cause of concrete deterioration and if this process is not understood and addressed, the structure will deteriorate unnecessarily and may be considered for replacement. “It is important to consider the total cost of each option before making a decision. Decisions are often made based on initial cost or construction cost and do not include the full societal cost which includes the cost of delays, congestion, and environmental costs. Informed decisions should be based on full costs.” (Dave Whitmore)
Corrosion of steel in concrete is a widespread issue with far-reaching effects on infrastructure, the environment, and the economy. Understanding the underlying causes, such as chloride infiltration and carbonation, is crucial for developing effective prevention and mitigation strategies.
Implementing solutions like embedded galvanic anodes or impressed current systems can significantly reduce the rate of corrosion of steel in concrete. Adopting high-quality materials, adhering to proper construction practices, and conducting regular inspections are all essential to avoid the detrimental effects of corrosion.
By proactively addressing corrosion, we can enhance the durability and safety of our infrastructure, protect the environment, and reduce economic costs. Ensuring our structures remain robust and resilient is not just an engineering challenge but a societal necessity.
For more insights and detailed solutions to combat corrosion, stay tuned to our blog and ensure your structures stand the test of time.

World of Concrete Announces the 2025 Innovative Product Award Winners : The third annual awards …

Pacific Ports Magazine October/November 2024 — It is with great pleasure that the Association o…

Vector Corrosion Technologies Wins ICRI Award of Excellence for Cathodic Protection Work on the Hist…